Bavarian Nordic Mpox Vaccine Receives WHO Approval for Teenagers
The adolescent mpox vaccine developed by Danish biotech company Bavarian Nordic has received approval from the World Health Organization (WHO). Less than a month ago, Bavarian’s Mpox vaccination for teenagers was approved by the EMA. According to the WHO, on October 8, “prequalification of the vaccine MVA-BN for persons aged 12-17 years” was given. The vaccine is authorized to provide active protection against adult smallpox, mpox, and associated orthopoxvirus infections and illnesses.
Mpox Vaccine from Bavarian Nordic Cleared by WHO for Adolescents, Shares Surge
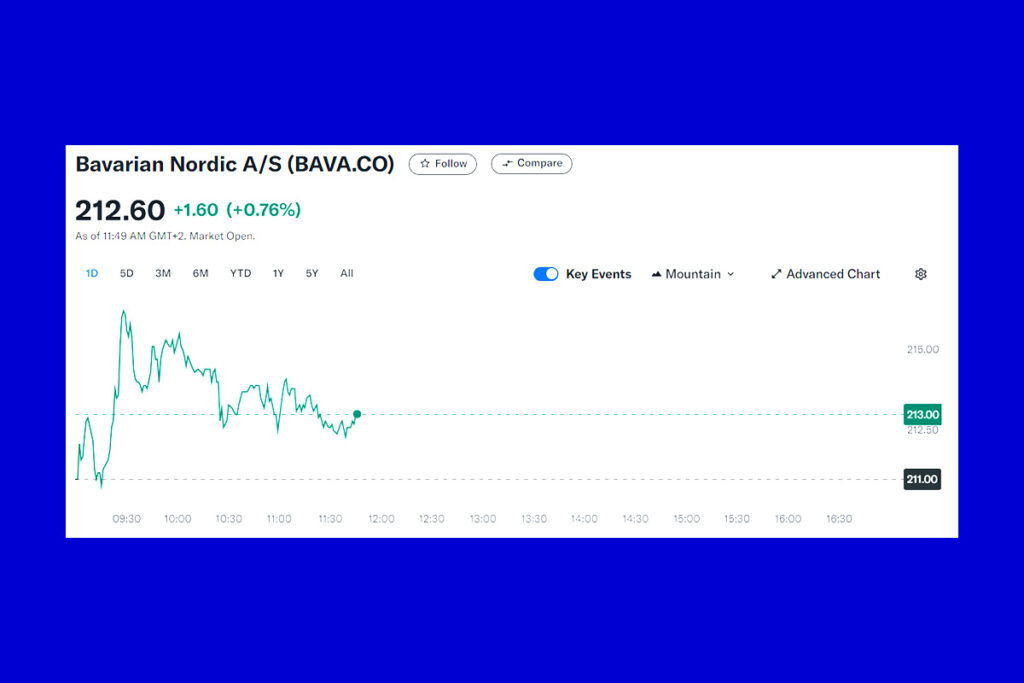
The mpox vaccine from Bavarian Nordic was approved by the World Health Organization on Monday for adolescents between the ages of 12 and 17, who are thought to be particularly susceptible to illness outbreaks that have raised concerns around the world. Following the spread of a novel strain of the virus from the Democratic Republic of the Congo to its neighbors, the WHO in August proclaimed mpox a worldwide public health emergency for the second time in two years.
Access to the vaccine is made easier for severely affected African nations by the United Nations agency’s approval of its use in September as the first adult mpox vaccination. In order to evaluate the vaccine’s safety among youngsters between the ages of two and twelve, the Danish biotech company is also getting ready to carry out clinical research, which could expand its application. In light of all these developments, Bavarian’s shares are on the rise today.
For more up-to-date crypto news, you can follow Crypto Data Space.



Leave a Reply